Alejandra Coronel-Zegarra '22
Major(s): Biochemistry, Management Economics
Year: Junior

Dipyrrin is an organic scaffold with the potential to form metal complexes. These complexes are explored for oxygen activation for uses in fuel cells or photodynamic therapy. In this research, dipyrrin was synthesized and coordinated with various metals to investigate its photochemical and electrochemical properties.

Major(s): Biochemistry, Management Economics
Year: Junior

Major(s): Microbiology, Pre-Med
Year: Junior

Assistant Professor of Chemistry, Ohio Wesleyan University
Dipyrrin is a photosensitizing agent that is known for forming flexible metal coordinations, which is of particular interest in cancer therapy and fuel cell production. The activation of oxygen using dipyrrin has allowed for a more efficient approach with cleaner byproducts in making fuel cells and a safer alternative to cancer treatments known as photodynamic therapy. In this research, dipyrrin was synthesized and coordinated with various metals to investigate its photochemical and electrochemical properties.
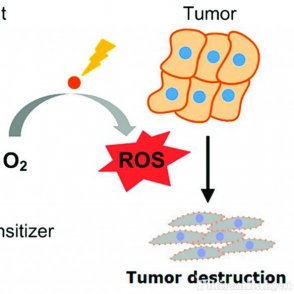
Photodynamic therapy (PDT) is one of the promising applications of synthesizing dipyrrin ligand scaffolds. It is an alternative to chemotherapy cancer treatment that targets tumors through injecting a chemical called a photosensitizer into the body. This chemical penetrates the tumor and enters an excited state when activated by UV light. This activation produces oxygen molecules in the tumor cells. This increased concentration of oxygen is toxic for the tumor cells, which results in apoptosis of the tumor cells specifically.
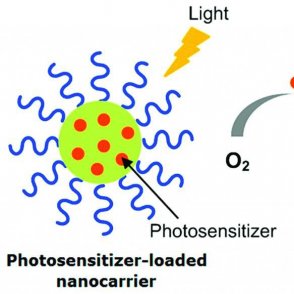
Overview of PDT
Hydrogen fuel cells have the ability to generate power with only water as a byproduct, making these fuel cells a much more eco-friendly source of energy than standard gasoline. Dipyrrin as a ligand is an electron reservoir; thus, utilizing this ligand in the conversion of oxygen in air to water, a process that is usually difficult, can help activate oxygen and reduce the strain on the metal in the scaffold.
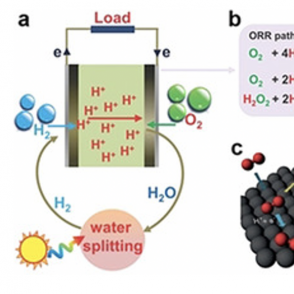
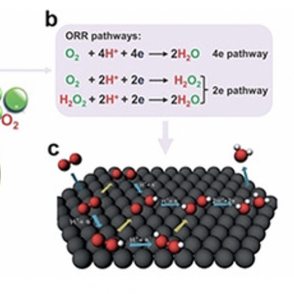
ORR Pathways in Fuel Cell Production
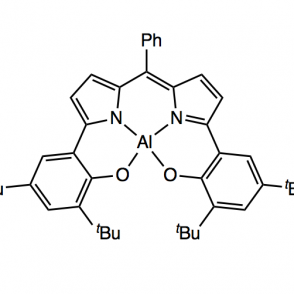
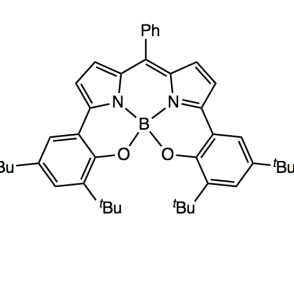
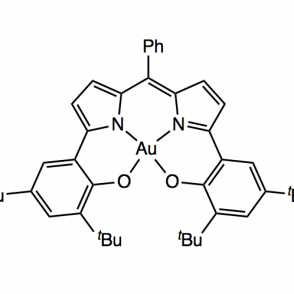
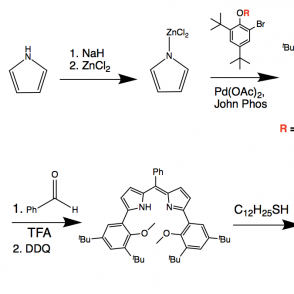
A redox active ligand scaffold, dipyrrin, was synthesized for the purpose of activating oxygen. The pathway of focus entailed a Negishi coupling followed by condensation with a phenyl aldehyde using pyrrole and di-tert-butyl phenol, using either methyl or silyl protecting groups. The use of these groups was investigated to explore a more efficient synthesis; as expected, the results indicated that the methyl protecting group was more effective due to sterics. Complexes were formed with the phenyl ligand derivative using aluminum (III) and boron coordinations. These organometallic compounds were analyzed using proton NMR, UV-Vis spectroscopy and electrochemistry.
A synthesis was done to produce a dipyrrin ligand scaffold phenyl derivative. The synthetic approach is shown on the right.
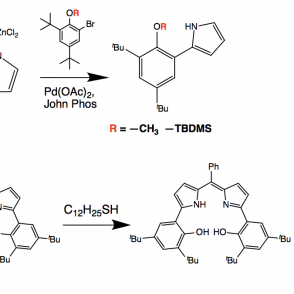
Upon synthesizing the dipyrrin ligand, it can undergo metalations for phenyl ligand derivatives using various metals, including Aluminum and Gold. A boron complex has also been investigated. These complexes can then be used in the activation of oxygen.